👋 If you need to download some data from the NIMH Data Arhive but do not have access to it, you’ve come to the right place!
Creating a Data Use Agreement for NIMH Datasets
Note: This tutorial uses the ABCD and HCP/CCF datasets as an example for illustration purposes.
Getting started : Download and fill out the most up-to-date Data Use Certification (DUC)
You can access the most recent version here. If the link doesn’t work, you can search Google for “NIMH Data Archive Data Use Certification” to get the most updated version.
Next, you need to fill out the form (don’t forget to add yourself as a recipient), and have Ted sign it.
One thing of note is under section 5. Other Recipient(s) on page 9, if there are not enough entries for all DUC Recipients, you can duplicate this page and use Adobe Acrobat Pro to modify page numbers and field labels. Steps for changing form fields are:
- Navigate to Prepare Form mode (you can find this under “Tools” > “Prepare Form”).
- Select each form field that has been duplicated and click the field’s properties (right-click > Properties).
- In the General tab, change the name of the field to something unique for each duplicated page.
Step 1 : Enter the RIS
You will need a valid PennKey for this step.
Research Inventory System is the web-based application for requesting, routing, and issuing various research related agreements.
Start by clicking on the system and providing your PennKey login. You should then see the following:

Step 2 : Create new data request
Go to DATA: If you want to receive/send data for research from/to an outside party, click here and click here.

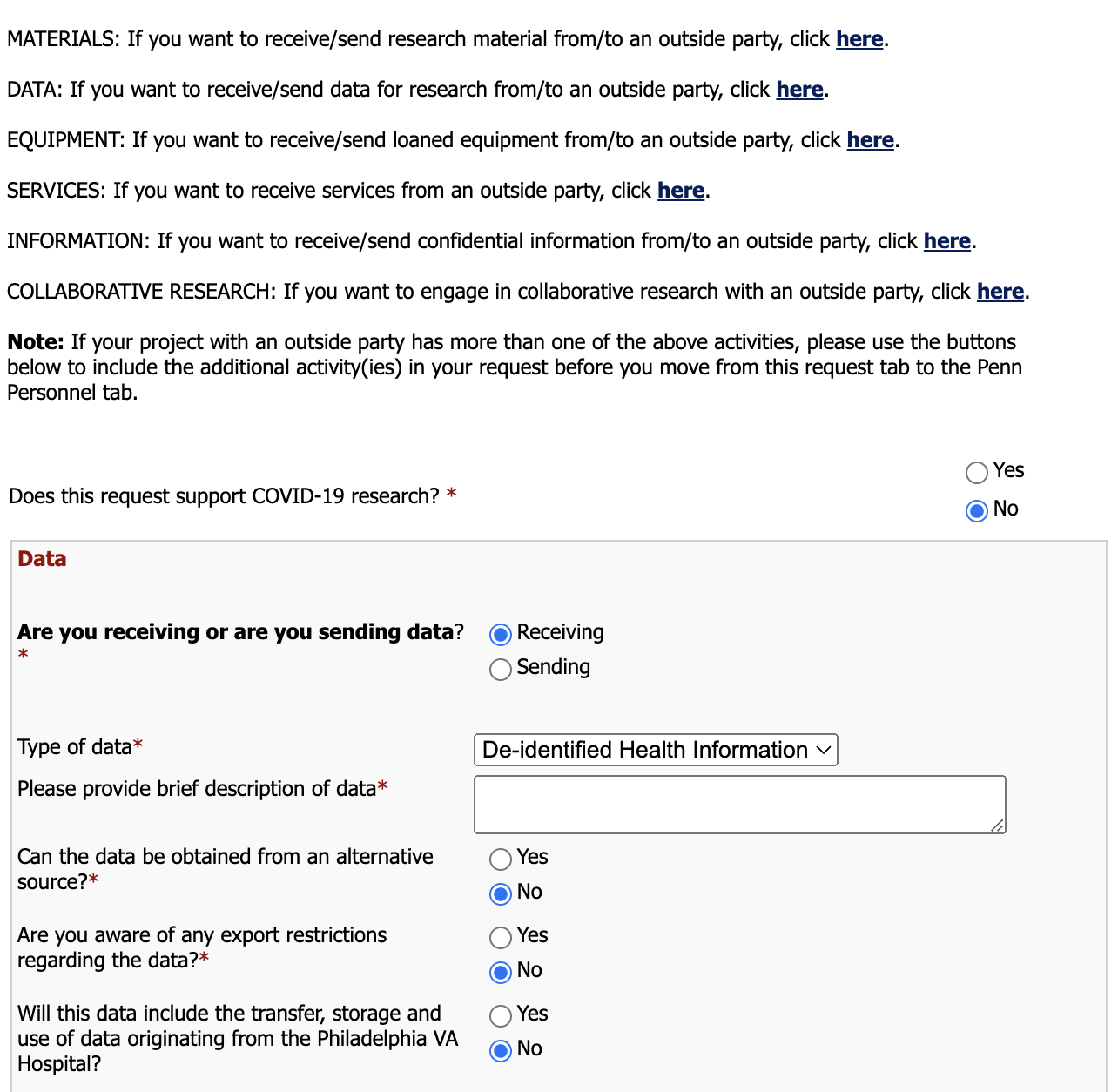
Are you receiving or are you sending data?
Since we are requesting data access, we select Receiving.
Type of data
In the case of ABCD/CCF, the type of data is De-identified health information.
Please provide brief description of data
Please provide basic description of your requested dataset.
Can the data be obtained from an alternative source?
In the case of ABCD/CCF, the answer is No.
Are you aware of any export restrictions regarding the data?
Select No.
Will this data include the transter, storage and use of data originating from the Philadelphia VA Hospital?
In the case of ABCD/CCF, the answer is No.
Step 3 : Select faculty member, business administrator and department

In our case, faculty member is Theodore Satterthwaite, and the department is Neuropsychiatry.

Consult the team for business administrator.
Step 4 : Upload research plan
Consult the team to come up with a research plan. This should be a short paragraph. You can either enter that plan in the blank field or upload it as an attachment.

This request is associated with
- Federally sponsored/federal flow through research
- Corporate sponsored clinical trial or human subjects research
- Foundations/other non-profit sponsored research
- Other corporate sponsored research
- Other
ABCD/CCF is funded by the National Institute of Mental Health (NIMH) which is a federal agency. Hence we select Federally sponsored/federal flow through research
Are your requests (materials, data, equipment, confidential information , services and/or collaboration) related or connected to a sponsored research agreement (SRA), a clinical trial agreement (CTA), a government or grant funded project, or will it be charged to a fund?
- Yes
- No
In the case of ABCD/CCF, the data request is for the purpose of scientific research. The research is neither related nor connected to a sponsored research agreement (SRA), a clinical trial agreement (CTA), a government or grant funded project. Hence we select No
What is the likelihood of an invention resulting from the research?
- May possibly result in an invention
- Is likely to result in an invention
- Is unlikely to result in an invention
In the case of ABCD/CCF, the data request is for the purpose of scientific research that would not lead to any invention.
Is the research related to any of your previous or pending disclosures of inventions?
- Yes
- No
In the case of ABCD/CCF, the data request is for the purpose of scientific research that is not related to any disclosures of inventions. Hence we select No
Modify as necessary for your specific requested dataset.
Step 5 : Fill out data information
Below is how you would fill out the data information for ABCD/CCF.
Modify as necessary for your specific requested dataset.
Step 6 : Upload data use agreement
Upload the signed and dated data use agreement form that you prepared at the beginning by clicking Upload outside party agreement.

Step 7 : Final submission
Select No for the question and you can click on submit.

Step 8: Wait for approval from the Office of Research Services
Just relax and wait for a final approval email to pop up in your inbox. The subject line would read like MTA/NMA (58028/00): Request fully executed.
Step 9: Contact the Lead Recipient
Per the new NDA policy, The only way to properly submit a DUC is for the Lead Recipient to upload the document to the appropriate DAR on their Data Permissions dashboard (https://nda.nih.gov/user/dashboard/data_permissions.html).
Now you should contact the Lead Recipient for the DUC to execute the following steps:
visit the Data Permissions dashboard (https://nda.nih.gov/user/dashboard/data_permissions.html)
click ‘Reapply for Access’ under the ‘Actions’ dropdown for the Data access request
locate the updated DUC file
upload the DUC to Data Access Request.
Once you receive the Permission Approved Email from NIMH, pat yourself on the back and enjoy a moment of relief. However, you are not yet done and please read on.
Accessing the Data
Accept the Terms of Data Use
After you’ve been granted access, you need to log in to the NDA website to review and accept terms of data use. The prompt window should look like below: 
Make sure you click on Accept.
Obtain AWS Credentials
Note 1: For security purposes, the AWS access key ID, AWS secret access key and AWS session token referenced below are all FAKE values. Please replace them with your real ID/key.
Note 2: This tutorial is partially adapted from the NDA’s tutorial How to Access S3 Objects.
Step 1: Download a copy of the NDA download manager
The command line downdownload manager is written in Java and allows for the automatic download of files. We can use wget to retrieve the program from the NIH web server.
wget https://ndar.nih.gov/jnlps/download_manager_client/downloadmanager.zip
Step 2: Unzip the download manager
Since the downloaded program is a zipped file, we would need to unzip the program to use the download manager.
unzip downloadmanager.zip
Step 3: Use the command-line download manager to generate temporary Amazon S3 security credentials
We specify the name of the output file awskeys.txt with the -g flag.
java -jar downloadmanager.jar -g awskeys.txt
You will be prompted for your ndar username and password, after which you will be able to find your keys in the file awskeys.txt
cat awskeys.txt
You will see something like below from your awskeys.txt file.
accessKey=xxxxx
secretKey=xxxxx
sessionToken=xxxxx
expirationDate=xxxxx
Step 4: Export the AWS S3 credentials to your working directory
For instance, navigate to ~/.aws/credentials and paste in the awskeys.txt according to the format below:
[NDAR]
aws_access_key_id = xxxxx[copy and paste the value of accessKey in `awskeys.txt`]
aws_secret_access_key = xxxxx[copy and paste the value of secretKey in `awskeys.txt`]
aws_session_token = xxxxx[copy and paste the value of sessionToken in `awskeys.txt`]
Now you should be good to go! Shoot us an email if you still run into issues.